As part of our semaglutide program, JY MedTech has successfully completed the registration of the semaglutide active pharmaceutical ingredient (API) Korean Drug Master File (K-DMF) in South Korea.
The API registration certificate has been issued by the Korean Ministry of Food and Drug Safety (MFDS)
Registration No.: 20260123-211-J-2117
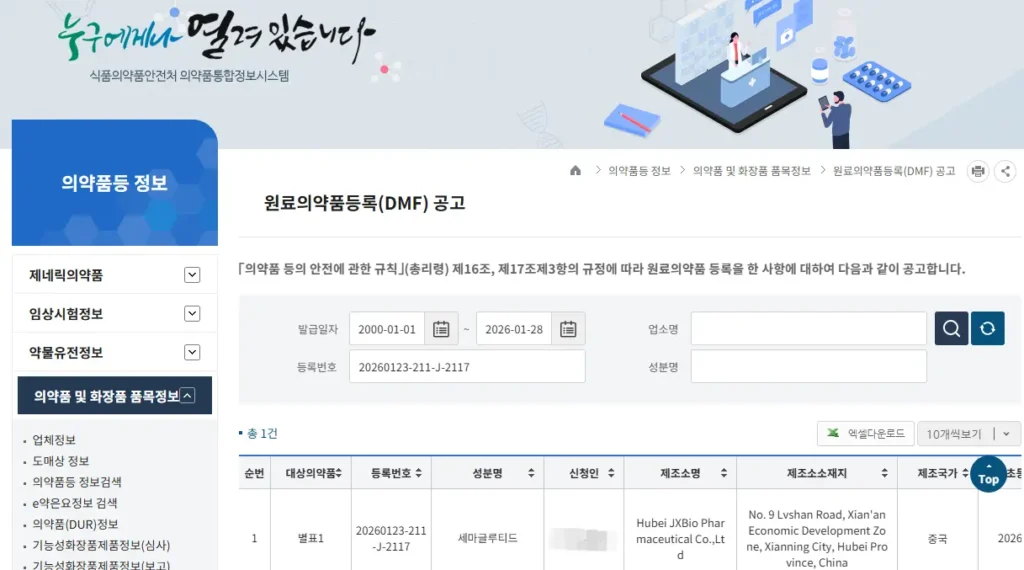
This milestone confirms that the semaglutide API K-DMF has been formally registered and certified by MFDS, marking a key step toward entering the Korean pharmaceutical market and supporting our overseas business expansion.
The successful K-DMF registration demonstrates our regulatory readiness and provides a solid foundation for partners advancing development and registration activities in South Korea. It reinforces our focus on disciplined compliance, dependable documentation, and scalable supply execution.
Onward with precision, speed, and quality.
#Pharmaceuticals #API #Semaglutide #KDMF #SouthKorea #JYMedTech #Regulatory #CDMO


0 Comments